|
Periodic Table - Correlation of Numerical Properties of Elements
Introduction More than 600 different forms of periodic tables have appeared in scientific literature during the last 130 years. One of the first was drawn by the Russian chemist Dimitri Mendeleyev on 1869. Mendeleyev is rightly seen as the discoverer of the periodic table because his own table was based on strictly scientific principles. The fact that Mendeleyev produced his periodic table 27 years before the discovery of the electron (Joseph Thomson, 1897) and 14 years before the discovery of the positively charged atomic nucleus (Ernest Rutherford, 1911), is amazing. The vertical sequences in the table are called groups, and the horizontal sequences are called periods. The elements within the same group are characterized by a striking similarity of their chemical properties, whereas a smooth transition of their numerical physical or chemical properties is almost always evident. This smooth transition allowed Mendeleyev to predict the properties of a number of "missing" elements, decades before their actual discovery.
Applet This applet allows the user to assess quickly a number of "numerical" properties of the elements, i.e. properties that can be described by a number. These properties have been classified in the following 4 groups: Chemical Properties: atomic number - atomic weight - radius (atomic) - radius (covalent) - radius (Van der Waals) - electronegativity (Pauling) - electronegativity (Allred) - electronegativity (absolute) - effective nuclear charge (Slater). Physical Properties: melting point - boiling point - density (20οC) - molar volume - thermal conductivity (27οC) - electric conductivity - coefficient of linear expansion - enthalpy of fusion - enthalpy of vaporization - enthalpy of atomization - Young modulus - rigidity modulus - bulk modulus. Electronic Properties: electron affinity - electron work function - 1st to 4th ionization energy. Miscellaneous Properties: abundance in earth crust - abundance in sea - abundance in sun - hardness (mineral) - hardness (Brinell). The group of properties can be selected by clicking on the corresponding radiobutton and the actual property by clicking on the buttons labeled as ">>" or "<<". The fill color of each element cell acquires a color shade indicative of the actual value of the selected property (white/orange/red color scale). By moving the cursor within the periodic table one can observe the actual value of the selected property on the upper part of the table. The actual units are also shown and occasionally information is included about the allotropic form of the element, to which the numerical value applies.
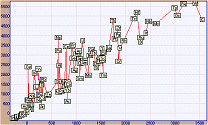
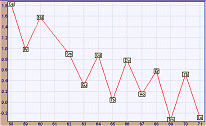
How to Create a Correlation Plot In order to create a correlation plot, i.e. a plot of the values of a property A versus the values of a property B, one has first to select these two properties in the corresponding windows. Then, the user has to select the elements to be plotted by clicking on the "Select Elements" button. Of course, numerical data for both properties selected for a given element must exist, otherwise the element is automatically excluded (its cell on the Periodic Table acquires the applet background color). By clicking on a cell the corresponding element can be selected or excluded. Cells of selected elements acquire a cyan color and those of excluded acquire a reddish color. For fast selection/exclusion of all elements you can click on the rectangles labeled "Select All" or "Exclude All". Once the elements of the correlation plot have been selected, the user must click on the button "Correlation Plot" to create the corresponding plot. Each point of the plot corresponds to an element, whose symbol appears in a small yellowish rectangle representing a single data point. A typical correlation plot of boiling points versus the melting points of all elements (of known m.p. and b.p.) is shown below (in reduced size). From this plot one concludes that there is some degree of correlation between these two physical properties. The plot of log(abundance in earth crust, ppm) versus atomic number exclusively for the lanthanides is shown below. The characteristic alternating trend (zig-zag) of abundance of lanthanides is obvious.
This applet allows the user to create a large number of correlation plots like those shown in these two examples and study the within group and period trends, as well.
Sources Most of the numerical values of the properties of the elements were obtained from: J. Emsley, "The Elements", 2nd ed., Oxford University Press, 1992. |